ENTRx is a biotech company that develops therapeutics targeted for human and veterinary ear diseases.
The company was founded by a practicing ENT and a pharmacist. ENTRx’s patented technology is a gel-like viscous suspension used to fill the ear canal, ensuring contact with all infected tissue.
OTITIS EXTERNA: AN IMPRECISE DIAGNOSIS
“Here is a good old-fashioned acute otitis externa. Is it fungus? Is it bacteria? Both? You’ll never know until they fail treatment and weeks have gone by.”
Matthew Branch, M.D.
CEO and Co-founder, ENTRx
OTITIS EXTERNA: ORIGINS
OTITIS EXTERNA: EPIDEMIOLOGY
One in 10 patients will suffer otitis externa in a lifetime.
90% of cases of otitis externa are due to bacteria.
10 % of otitis externa are due to fungus.
Fungal otitis externa is more common when a patient has already been treated with antibiotic drops and when symptoms last longer than one week.
BACTERIA OR FUNGUS:
WHY IS OTITIS EXTERNA HARD TO TREAT?
A Diagnosis of Guesswork
Co-pathogens: Bacterial, fungal, or mixed
NOT standard of care to culture on the first visit
Fungus RARELY diagnosed until therapy fails -- often WEEKS after initial symptoms
Visually difficult to differentiate infections
Clogged and Swollen Canals:
A plumbing problem
No continuous contact with the infected tissue
Sparse and inconsistent coverage
Rapid egress from the ear canal
Subtherapeutic concentrations in the microenvironment
Drops Drip Out
Edema, bacterial pus, waxy debris, and fungal mass
Difficult drop delivery
TREATMENT CHALLENGES
Initial encounter rarely yields correct diagnosis of fungus
No FDA-approved treatment for fungus in the ear
Off-label treatments not ideal, often requiring repeat office visits to debride excipients from canal
Development of fungal otitis externa as a secondary infection
Is It Fungus?
Hard to aim drops into clogged and swollen canal
Difficult for older patients with poor dexterity
Tedious: self-administered fourteen times over one week
Patients rarely use drops as prescribed
Poor Patient Compliance
Home
Office
OUR LEADERSHIP
Ronald Kuppersmith, M.D., MBA
Advisor
Ron is the CEO and co-founder of ENT Specialty Partners, a multi-site and full-service private practice in Texas. He is a proven leader within the specialty, having served as the President of the American Academy of Otolaryngology-Head and Neck Surgery, President of the Texas Association of Otolaryngology, and as a Professor of Surgery at the Texas A&M Health Science Center College of Medicine.
Ron has extensive experience working closely with management of health service, medical device and pharmaceutical companies, non-profit organizations, and investors in a variety of capacities including board member, founder, investor, advisor and in operational roles. Ron is the Chief Medical Advisor for Cyrano Therapeutics, a former board member of Spirox (acquired by Entellus), former board advisor of Entellus (acquired by Stryker), and a former board member of Arrinex (acquired by Stryker).
Ron trained in Otolaryngology-Head and Neck Surgery at Baylor College of Medicine in Houston. He has a BA in Economics and MD from the University of Michigan and an MBA from University of Washington.
ENT401 is a viscous, hydrophobic carrier for a wide variety of APIs, allowing exposure and easy absorption across any epithelial or mucosal surface.
The elegant nature of the ENT401 vehicle is in its minimalist design. This approach avoids the retention of excipients common in other products designed for use on the skin. These products routinely desiccate and adhere, necessitating multiple in-office painful debridements.
OUR PLATFORM
Our technology is based on four elements:
Suspend the APIs in a viscous carrier
Distribute them throughout the treatment area for wide exposure and absorption
Create a hydrophobic environment that inhibits the survival of bacteria or fungus
Remove barriers to API exposure by dissolving and dislodging persistent cerumen or dry skin
Application Opportunities Beyond ENT401
Otorhinolaryngology
Sinusitis
Genitourinary
Cystitis, urethritis, ureteritis
Ophthalmology
Blepharitis, conjunctivitis
Wound care
OB-GYN
Vaginitis, cervicitis
OUR SOLUTION: ENT401
With ENT401, all otitis externa can be treated whether bacterial or fungal. ENT401 will be the first FDA-approved single dose treatment for otitis externa with time-tested antibacterial, antifungal, and anti-inflammatory APIs to treat pain, itch, and swelling.
The current seven-day course of drops often fails. In comparison, ENT401 is a single dose treatment for otitis externa of bacterial or fungal origin, administered by a health care provider with relief of symptoms within 2-3 days and less than 1% persistent disease.
NEW SINGLE-DOSE FORMULATION
A proprietary, viscous suspension that delivers 3 FDA-approved APIs
OUR PIPELINE
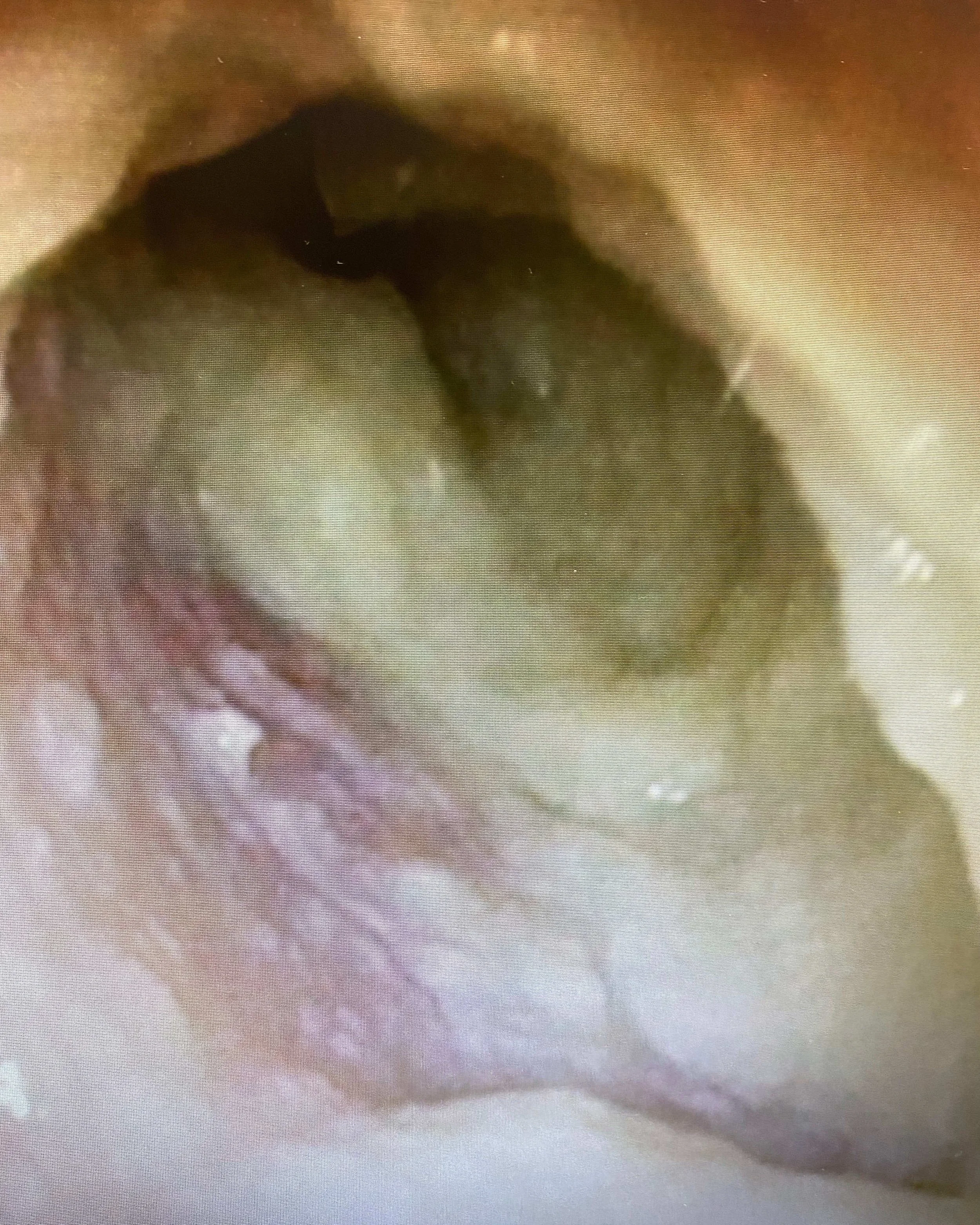
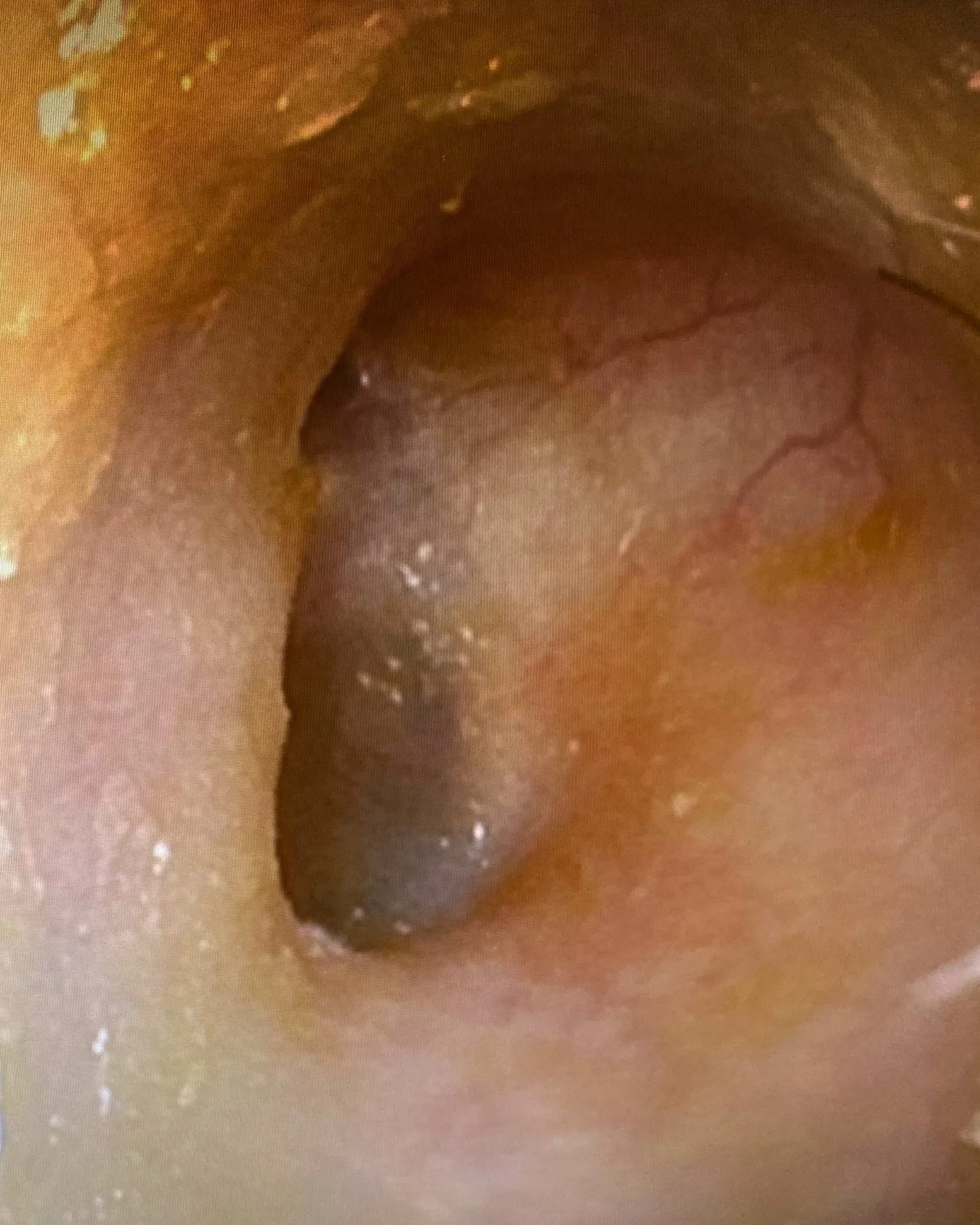
SEE ENT401 IN ACTION
BEFORE TREATMENT
AFTER TREATMENT (7 DAYS)